@rustystuds. I agree, but the diagram kind of assumes double pane, and a partial vacuum, on the side opposite the air, so I figure there is no real particle activity to report there. Just radiation across the interstitial space.
Also, my entire cubicle is, in fact, double glazed (save for the spider plant jade-tree interior green roof), but my cubicle-mate is lithe and comely, so it's a non-issue.
Bah, I have no quick method of image hosting so my sketch will have to wait.
@hb
Everything is correct except for one thing.
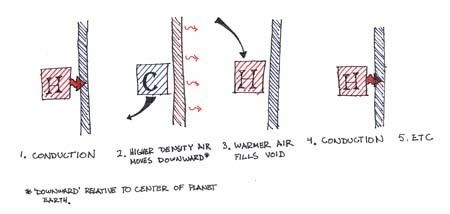
Referring to the two fluid particles that are in physical contact that you have labeled as "conductive transfer," let us call the one on the left particle A and the one on the right particle B.
Particle A is experiencing pure convection because it is transferring heat from a fluid particle (itself) to another fluid particle (particle B). This is a textbook example of convection.
Particle B is receiving heat from another fluid (particle A)- so it is receiving heat convectively. However, since it is in physical contact with the glazing it is transferring heat to the glazing via conduction.
This dual behavior implies that this particular particle is the convection-conduction interface and is a transition between two different types of heat transfer. Is it a fluid particle? Yes, so it must be convection. Is it physically touching another object? Yes, so it must be conduction as well.
A lot of people (myself included) lump this interface in with the general term of convection since it is a fluid particle to begin with. It is one of the small nuances of thermodynamics- unfortunately there are a lot of those.
As for the interstitial space:
The interior (hatched) region of the glazing is transferring heat across its section via pure conduction from the no-slip region particle until it reaches the interstitial surface. If it is a vacuum, it will transfer to the opposite glazing surface via radiation. If it is gas filled, another boundary layer will exist and heat will be transferred similarly through a no-slip regionvia conduction, to the interstitial gas via convection, to the opposite glazing's no slip region via convection, and then to the other glazing via conduction.
Thermodynamics can get pretty ugly, no doubt about that.
I always figured that convection was synonymous with mass transfer, or that convective heat transfer is a result of mass transfer. So that a region of air at temperature A would move to a location at temperature B and replace the air there, thus raising the temperature of that region, and the temperature B air would move out of the way, but no inter-particle kinetic interaction is taking place (except to give the region of air a statistically uniform overall temperature). So in the diagram, convection gets the particle (and its energy) to the no-slip particle, but that's all it does, transfer that mass. Getting the energy from the particle to the 'no-slip' particle requires a kinetic, thus conductive, transfer.
@cjk5027 - "The interior (hatched) region of the glazing is transferring heat across its section via pure conduction from the no-slip region particle until it reaches the interstitial surface."
just a thought experiment - since glass is an amorphous non-crystalline solid achieved from increasing viscosity, is there any conductive transfer within itself?
Personally, I think cjw explains it well and it's all pretty understandable. What I don't understand is why in the world cjw, after 2 engineering degrees, wants to become an architect. Unless you get a job in a cutting edge office, you will rarely get the opportunity to make sufficient use of your knowledge. I won't even go into the other drawbacks of being an architect.
Mass transfer is something else altogether. That would typically be used for analyzing something like a cycle (combustion, refrigeration), a process (e.g. chemical refining), or flow movement (e.g. water through a network of pipes).
Convection doesn't involve mass transfer. Take a blow dryer, turn it on hot, and blow it on your arm from a safe distance. Your arm will heat up, but will not become more massive nor replace the mass of your arm with the mass of the hot air due to this convection.
bk:
I got my bachelor and master of ae simultaneously in the same amount of time as a bachelors, so I only really consider that one educational experience.
Much like our friend the convective-conductive interface (nerd joke!), after getting my M.Arch I'd like to get into that gray area between architecture and engineering/ design and performance. At the moment I am only playing with a half deck, so to speak.
I would be very happy if I could get into healthcare design, science/tech facility design, enclosure design, or forensics/ disaster recovery. It seems that someone who is versed in design and performance can contribute a lot in those three fields.
@cjw5027:
In the blow dryer example: of course there is not mass transfer between hot air and arm, but isn't the arm in the same diagrammatic position as our pane of glass? So, the fan of the blow dryer is transferring a hot air mass to the surface of the arm where it replaces some room temperature air. Then this mass of warm air conducts its heat to the skin of the arm? Isn't convection about movements regions in a fluid mass due to temperature differential? In a passive case this would be driven by density differences, but in the case of a blow dryer it is mechanically driven.
Fluid regions of air mass are shown as boxes for the sake of the diagram.
On the subject of blow dryers, don't you think most of the heating effect comes from thermal radiation, and the air movements is more about inducing evaporation? This is a better example to think about radiation in fact, given how critical it is to point the radiating surface at the surface to be heated.
So, isn't a convection current a kind of heat pump, driven by a consistent temperature difference? An entirely fluid machine? It is a kind of an emergent fluid dynamical organization. This is just the kind of thing that people like to talk about in architecture school (I think it's great that you have opted to become an architect).
Lastly, on the larger subject of mass transfers, maybe this is a good time to discuss HEAT PUMPS pursuant to our exploration of EVAPORATIVE CHILLERS!
It may help to envision the surface of the glass as very densely packed particles rather than a uniform mass. There are going to be attractive intermolecular forces between the molecules of air and the molecules of glass, causing this single-molecule thick no slip layer to build up.
The same particles of air may very well be be stuck to the glazing surface for years, one particle doesn't supplant another because it has more energy (heat)- it merely passes its energy on to it.
In order to move one of the no-slip air molecules from the glass surface, the intermolecular forces have to be overcome by other forces, and there are a variety of ways in which this could occur. The act of transferring heat does not generate a force alone. If an air particle was traveling fast due to being in a higher energy state, its impact could have enough force to dislodge the 'stuck' molecule and the initially fast-moving particle could take its place.
The blow dryer example I used was intended to focus solely on the heated air convection. Half of the battle of thermodynamics is realizing what the bounds of your system/ frame of reference are- if you want to have a big enough frame, a blow dryer can have all three modes of heat transfer at different points.
Convection currents are not heat pumps- a heat pump is an active (i.e. energy is put into it to make it work) mechanical system with many different parts and several different stages.
Work is starting to pick up, but this weekend I will get off my hindquarters and find an image hosting site. I think some sketches would help clarify some of these ideas much more clearly.
If this is what students like to talk about and view it as a valuable part of their education, then I hope admissions committees see some sort of value in the AE background and are willing to admit and to give some financial aid for a broke student-hopeful like myself :]
I am assuming those air particles that are in the no-slip zone are effectively part of the glass, from the standpoint of convection. I am then saying the free moving particles or air are conducting their energy to this 'glass-no slip layer' entity. Convection moves free particles into a location adjacent, and conduction transfers the energy.
A pot of boiling water is a common model of convection. It is actively energized via a flame or some other active source, and it moves heat from point a point A to a point B via aggregate particle flow. Isn't this a kind of abstract, uncontained heat engine? A Carnot engine moving heat from one place to another across a difference gradient?
Following that, if we can identify a active energy input creating the temperature differential in the room air then we have an analogous case no? And, in our example case I am guessing the warm parts of the room are actively heated by some system (wood stove, electric elements, steam-heat radiators), so isn't this also a heat pump in the loose sense? I guess this extends the bounds of the defined system a bit.
I think you have an understanding of the core concept correctly, but there are a few semantic issues.
Even though the free moving air and no-slip air particles are physically touching, when they transfer heat this process still has to be considered convection because they are both fluid particles.
The gray area comes when the no-slip particle and glazing particles are physically touching, you can consider it either convection (because a fluid is involved) or conduction (because they are physically touching and both are not liquid).
This double-take on the rules means that it must be considered in another category- it is a convection-conduction interface. Pure convection only exists between fluids (e.g. between air and car exhaust). Every other fluid must go through a convective-conductive interface before transferring heat to a solid.
re: heat pump
The convection current is not a heat pump, it is just a convection current- heat pumps and engines have energy (heat) wells and a transfer media we call a working fluid.
The best example of a heat pump would be a geothermally-based underfloor system. If you have a grasp of how heat flows back and forth between the room, the fluid in the floor coils, and the ground in the summer and winter then that is the perfect example of how a heat pump works.
I'll get into that more once I have some more time away from work and can produce some process sketches- this weekend, most likely.
Annoying reality:
In thermodynamics, if you alter your reference frame (called a control volume or control system) enough you can pretty much justify any situation to explain a hypothesis.
I found that with many students I tutored, setting this reference frame was the hardest thing to do and quite honestly comes with practice and experience. However, learning how to set this frame of reference is an implicit prerequisite for almost every other thermodynamic theory.
Setting the reference frame is the first thing any engineering thermodynamics class will cover. It is incredibly boring, but incredibly critical to the entire process. I'll cover this in some sketches as well.
Then, can we then define the system as an average living room in Northeast, USA, on a seasonable day in early March, containing atmospheric air, one double glazed window to the exterior, a steam-heat radiator opposite the window and adiabatic walls?
Air is the working fluid, the window pane is the cold sink, a domestic heat source is the heat source. Expansion and contraction of air due to temperature difference drive cyclical action which results in moving heat energy from point A (near radiator) to point B (near window)?
Perhaps the problem is that there is no real opportunity to extract work from this system? But, of course we could, say, drive an low-friction windmill-type apparatus and extract a small amount of work.
(Aside: If this windmill was charging a low-wattage microprocessor capable of doing some meaningful data-based work for another high-energy thermodynamic system (say working as a virtual governor mechanism) then maybe we could say we are extracting a large amount of meaningful work from this system.)
Or is the requirement that the working fluid needs to undergo a phase change in the work cycle in order to be a true heat hump? This isn't the case in a ground-source heat pump.
So maybe it's a simply a function of there being no sensible material boundary to the system that makes it not qualify as a heat pump?
I know this thread is now getting a little out there, but I actually feel pretty strongly about this conceptually. If we can't allow that the above mentioned system is a heat-pump, perhaps we can include them both in a larger category of cyclical energy transport systems via fluid media?
That is quite potentially the most abstract, theoretical, never-to-be-used-in-industry usage of the term heat pump that I have ever encountered- but given a few extra components to get work in and out of the system and a few other relatively minor assumptions, I could agree that this entire room behaves like a heat pump with air as a working fluid.
About to leave work- explanation to come tonight or sometime tomorrow.
I have one question regarding this and you can school me on this cjw5027 if you want.
But could the problem also be related to pressure? As in, there's a difference between the pressures of interior air and exterior air.
Stopping drafts and wind gusts is one thing. But a constant pressure difference (air-tightness) is a more complex issue. Rather than looking at this purely thermodynamically, is there an argument for simple air mixing via a pressure gradient?
Well as of today (according to the GSAPP) I do not make the cut to 'school' anyone :) It was my last choice and I am still keeping my fingers crossed for the other 5, but I digress.
Seeing how this question turned out to be one of a practical nature rather than theoretical as I had initially interpreted, it could be the result of any number of problems. I do not want to say for certain, but it is quite possible- albeit a much more complicated scenario.
The pressure differential would exist due to the result of something else failing; an underperforming HVAC system, poor window construction, drastic changes in environmental conditions, and so on. It could even have nothing to do with the building itself, but maybe other construction in the area influenced air flow around this particular window.
So is there a simple argument? I'd lean towards no. It's out of my pay grade, anyway.
I have to say- if I get in somewhere, I would love to help teach a building science course. This has been a very fun discussion (for me at least).
The area near the window is cold, why?
is this area cold in the summer as well?
It gets salty in the summer. Courtesy of you chubby, perspiring cubiclemate.
@rustystuds. I agree, but the diagram kind of assumes double pane, and a partial vacuum, on the side opposite the air, so I figure there is no real particle activity to report there. Just radiation across the interstitial space.
Also, my entire cubicle is, in fact, double glazed (save for the spider plant jade-tree interior green roof), but my cubicle-mate is lithe and comely, so it's a non-issue.
Bah, I have no quick method of image hosting so my sketch will have to wait.
@hb
Everything is correct except for one thing.
Referring to the two fluid particles that are in physical contact that you have labeled as "conductive transfer," let us call the one on the left particle A and the one on the right particle B.
Particle A is experiencing pure convection because it is transferring heat from a fluid particle (itself) to another fluid particle (particle B). This is a textbook example of convection.
Particle B is receiving heat from another fluid (particle A)- so it is receiving heat convectively. However, since it is in physical contact with the glazing it is transferring heat to the glazing via conduction.
This dual behavior implies that this particular particle is the convection-conduction interface and is a transition between two different types of heat transfer. Is it a fluid particle? Yes, so it must be convection. Is it physically touching another object? Yes, so it must be conduction as well.
A lot of people (myself included) lump this interface in with the general term of convection since it is a fluid particle to begin with. It is one of the small nuances of thermodynamics- unfortunately there are a lot of those.
As for the interstitial space:
The interior (hatched) region of the glazing is transferring heat across its section via pure conduction from the no-slip region particle until it reaches the interstitial surface. If it is a vacuum, it will transfer to the opposite glazing surface via radiation. If it is gas filled, another boundary layer will exist and heat will be transferred similarly through a no-slip regionvia conduction, to the interstitial gas via convection, to the opposite glazing's no slip region via convection, and then to the other glazing via conduction.
Thermodynamics can get pretty ugly, no doubt about that.
I always figured that convection was synonymous with mass transfer, or that convective heat transfer is a result of mass transfer. So that a region of air at temperature A would move to a location at temperature B and replace the air there, thus raising the temperature of that region, and the temperature B air would move out of the way, but no inter-particle kinetic interaction is taking place (except to give the region of air a statistically uniform overall temperature). So in the diagram, convection gets the particle (and its energy) to the no-slip particle, but that's all it does, transfer that mass. Getting the energy from the particle to the 'no-slip' particle requires a kinetic, thus conductive, transfer.
@cjk5027 - "The interior (hatched) region of the glazing is transferring heat across its section via pure conduction from the no-slip region particle until it reaches the interstitial surface."
just a thought experiment - since glass is an amorphous non-crystalline solid achieved from increasing viscosity, is there any conductive transfer within itself?
oh - great info you're presenting - thanks.
gah! not conduction - convection...
Personally, I think cjw explains it well and it's all pretty understandable. What I don't understand is why in the world cjw, after 2 engineering degrees, wants to become an architect. Unless you get a job in a cutting edge office, you will rarely get the opportunity to make sufficient use of your knowledge. I won't even go into the other drawbacks of being an architect.
hbrain:
Mass transfer is something else altogether. That would typically be used for analyzing something like a cycle (combustion, refrigeration), a process (e.g. chemical refining), or flow movement (e.g. water through a network of pipes).
Convection doesn't involve mass transfer. Take a blow dryer, turn it on hot, and blow it on your arm from a safe distance. Your arm will heat up, but will not become more massive nor replace the mass of your arm with the mass of the hot air due to this convection.
bk:
I got my bachelor and master of ae simultaneously in the same amount of time as a bachelors, so I only really consider that one educational experience.
Much like our friend the convective-conductive interface (nerd joke!), after getting my M.Arch I'd like to get into that gray area between architecture and engineering/ design and performance. At the moment I am only playing with a half deck, so to speak.
I would be very happy if I could get into healthcare design, science/tech facility design, enclosure design, or forensics/ disaster recovery. It seems that someone who is versed in design and performance can contribute a lot in those three fields.
@cjw5027:
In the blow dryer example: of course there is not mass transfer between hot air and arm, but isn't the arm in the same diagrammatic position as our pane of glass? So, the fan of the blow dryer is transferring a hot air mass to the surface of the arm where it replaces some room temperature air. Then this mass of warm air conducts its heat to the skin of the arm? Isn't convection about movements regions in a fluid mass due to temperature differential? In a passive case this would be driven by density differences, but in the case of a blow dryer it is mechanically driven.
Fluid regions of air mass are shown as boxes for the sake of the diagram.
On the subject of blow dryers, don't you think most of the heating effect comes from thermal radiation, and the air movements is more about inducing evaporation? This is a better example to think about radiation in fact, given how critical it is to point the radiating surface at the surface to be heated.
So, isn't a convection current a kind of heat pump, driven by a consistent temperature difference? An entirely fluid machine? It is a kind of an emergent fluid dynamical organization. This is just the kind of thing that people like to talk about in architecture school (I think it's great that you have opted to become an architect).
Lastly, on the larger subject of mass transfers, maybe this is a good time to discuss HEAT PUMPS pursuant to our exploration of EVAPORATIVE CHILLERS!
are u guys going to charge nypencil tuition cause he is getting a better eduction on archinect than at harvard or wherever he goes. Just saying.
hb
It may help to envision the surface of the glass as very densely packed particles rather than a uniform mass. There are going to be attractive intermolecular forces between the molecules of air and the molecules of glass, causing this single-molecule thick no slip layer to build up.
The same particles of air may very well be be stuck to the glazing surface for years, one particle doesn't supplant another because it has more energy (heat)- it merely passes its energy on to it.
In order to move one of the no-slip air molecules from the glass surface, the intermolecular forces have to be overcome by other forces, and there are a variety of ways in which this could occur. The act of transferring heat does not generate a force alone. If an air particle was traveling fast due to being in a higher energy state, its impact could have enough force to dislodge the 'stuck' molecule and the initially fast-moving particle could take its place.
The blow dryer example I used was intended to focus solely on the heated air convection. Half of the battle of thermodynamics is realizing what the bounds of your system/ frame of reference are- if you want to have a big enough frame, a blow dryer can have all three modes of heat transfer at different points.
Convection currents are not heat pumps- a heat pump is an active (i.e. energy is put into it to make it work) mechanical system with many different parts and several different stages.
Work is starting to pick up, but this weekend I will get off my hindquarters and find an image hosting site. I think some sketches would help clarify some of these ideas much more clearly.
If this is what students like to talk about and view it as a valuable part of their education, then I hope admissions committees see some sort of value in the AE background and are willing to admit and to give some financial aid for a broke student-hopeful like myself :]
I am assuming those air particles that are in the no-slip zone are effectively part of the glass, from the standpoint of convection. I am then saying the free moving particles or air are conducting their energy to this 'glass-no slip layer' entity. Convection moves free particles into a location adjacent, and conduction transfers the energy.
A pot of boiling water is a common model of convection. It is actively energized via a flame or some other active source, and it moves heat from point a point A to a point B via aggregate particle flow. Isn't this a kind of abstract, uncontained heat engine? A Carnot engine moving heat from one place to another across a difference gradient?
Following that, if we can identify a active energy input creating the temperature differential in the room air then we have an analogous case no? And, in our example case I am guessing the warm parts of the room are actively heated by some system (wood stove, electric elements, steam-heat radiators), so isn't this also a heat pump in the loose sense? I guess this extends the bounds of the defined system a bit.
I think you have an understanding of the core concept correctly, but there are a few semantic issues.
Even though the free moving air and no-slip air particles are physically touching, when they transfer heat this process still has to be considered convection because they are both fluid particles.
The gray area comes when the no-slip particle and glazing particles are physically touching, you can consider it either convection (because a fluid is involved) or conduction (because they are physically touching and both are not liquid).
This double-take on the rules means that it must be considered in another category- it is a convection-conduction interface. Pure convection only exists between fluids (e.g. between air and car exhaust). Every other fluid must go through a convective-conductive interface before transferring heat to a solid.
re: heat pump
The convection current is not a heat pump, it is just a convection current- heat pumps and engines have energy (heat) wells and a transfer media we call a working fluid.
The best example of a heat pump would be a geothermally-based underfloor system. If you have a grasp of how heat flows back and forth between the room, the fluid in the floor coils, and the ground in the summer and winter then that is the perfect example of how a heat pump works.
I'll get into that more once I have some more time away from work and can produce some process sketches- this weekend, most likely.
Annoying reality:
In thermodynamics, if you alter your reference frame (called a control volume or control system) enough you can pretty much justify any situation to explain a hypothesis.
I found that with many students I tutored, setting this reference frame was the hardest thing to do and quite honestly comes with practice and experience. However, learning how to set this frame of reference is an implicit prerequisite for almost every other thermodynamic theory.
Setting the reference frame is the first thing any engineering thermodynamics class will cover. It is incredibly boring, but incredibly critical to the entire process. I'll cover this in some sketches as well.
Then, can we then define the system as an average living room in Northeast, USA, on a seasonable day in early March, containing atmospheric air, one double glazed window to the exterior, a steam-heat radiator opposite the window and adiabatic walls?
Air is the working fluid, the window pane is the cold sink, a domestic heat source is the heat source. Expansion and contraction of air due to temperature difference drive cyclical action which results in moving heat energy from point A (near radiator) to point B (near window)?
Perhaps the problem is that there is no real opportunity to extract work from this system? But, of course we could, say, drive an low-friction windmill-type apparatus and extract a small amount of work.
(Aside: If this windmill was charging a low-wattage microprocessor capable of doing some meaningful data-based work for another high-energy thermodynamic system (say working as a virtual governor mechanism) then maybe we could say we are extracting a large amount of meaningful work from this system.)
Or is the requirement that the working fluid needs to undergo a phase change in the work cycle in order to be a true heat hump? This isn't the case in a ground-source heat pump.
So maybe it's a simply a function of there being no sensible material boundary to the system that makes it not qualify as a heat pump?
I know this thread is now getting a little out there, but I actually feel pretty strongly about this conceptually. If we can't allow that the above mentioned system is a heat-pump, perhaps we can include them both in a larger category of cyclical energy transport systems via fluid media?
Thoughts, diagrams welcome.
You two get an award for dorks of the year :)
Can we go back to talking about magnets?
I accidentally wrote heat hump in that last post.
Magnets?
Hmm.
Question. Is induction a convection, conduction or radiative process?
Wow, I had to sketch that out.
That is quite potentially the most abstract, theoretical, never-to-be-used-in-industry usage of the term heat pump that I have ever encountered- but given a few extra components to get work in and out of the system and a few other relatively minor assumptions, I could agree that this entire room behaves like a heat pump with air as a working fluid.
About to leave work- explanation to come tonight or sometime tomorrow.
Suspense!
YES!
And to make rusty and glitter happy:
Induction is a magnetically induced process.
I kid you not.
I know it is. It technically is all three processes at once.
Without earth being a gigant fridge magnet, we wouldn't have much of an atmosphere, and thus neither the sticky air on the glass surface.
Magnets. Is there anything they don't do?
Are you inside or outside?
You should contact Cardinal Glass...and get the real poop!
This thread could have ended with this advice: wear layers.
Mrs Hoover, about Principal Skinner (behind his back): "Always with the magnets . . ."
I have one question regarding this and you can school me on this cjw5027 if you want.
But could the problem also be related to pressure? As in, there's a difference between the pressures of interior air and exterior air.
Stopping drafts and wind gusts is one thing. But a constant pressure difference (air-tightness) is a more complex issue. Rather than looking at this purely thermodynamically, is there an argument for simple air mixing via a pressure gradient?
GC
Well as of today (according to the GSAPP) I do not make the cut to 'school' anyone :) It was my last choice and I am still keeping my fingers crossed for the other 5, but I digress.
Seeing how this question turned out to be one of a practical nature rather than theoretical as I had initially interpreted, it could be the result of any number of problems. I do not want to say for certain, but it is quite possible- albeit a much more complicated scenario.
The pressure differential would exist due to the result of something else failing; an underperforming HVAC system, poor window construction, drastic changes in environmental conditions, and so on. It could even have nothing to do with the building itself, but maybe other construction in the area influenced air flow around this particular window.
So is there a simple argument? I'd lean towards no. It's out of my pay grade, anyway.
I have to say- if I get in somewhere, I would love to help teach a building science course. This has been a very fun discussion (for me at least).
Block this user
Are you sure you want to block this user and hide all related comments throughout the site?
Archinect
This is your first comment on Archinect. Your comment will be visible once approved.